|
| ||||||||||||||||||||||||||||||||||||
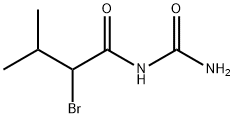
CAS号:496-67-3
|
英文名称:1-(2-Bromoisovaleryl)urea
分子式
分子量
EINECS号
MDL
Smiles
InChIKey
乙二醇化学百科
基本信息
物化性质
安全信息
生产及用途
Bromisoval (Bromovalerylurea, Isobromyl, Bromaral, BRN 1773255)是一种具有催眠、镇静效果的bromoureide类化合物。具有消炎活性。 Bromisoval (BU) suppresses nitric oxide (NO) releasing and proinflammatory cytokine expression in lipopolysaccharide (LPS)-treat BV2 cells, a murine microglial cell line. Bromisoval suppresses LPS-inducing phosphorylation of signal transducer and activator of transcription 1 (STAT1) and expression of interferon regulatory factor 1 (IRF1). The Janus kinase 1 (JAK1) inhibitor filgotinib suppresses the NO release much more weakly than that of Bromisoval, although filgotinib almost completely prevents LPS-inducing STAT1 phosphorylation. Knockdown of JAK1, STAT1, or IRF1 does not affect the suppressive effects of Bromisoval on LPS-inducing NO. A combination of Bromisoval and filgotinib synergistically suppress the NO releasing. The mitochondrial complex I inhibitor rotenone, which does not prevent STAT1 phosphorylation or IRF1 expression, suppresses proinflammatory mediator expression less significantly than Bromisoval. Bromisoval and rotenone reduce intracellular ATP (iATP) levels to a similar extent. A combination of rotenone and filgotinib suppress NO release in LPS-treated BV2 cells as strongly as Bromisoval.
Bromisoval (Bromvaletone) and carbromal are the most potent central depressants within each series. Depressant activities (ISD 50 values) and acute toxicities (LD 50 values) in male mice after intraperitoneal injection of Bromisoval are 0.35 (0.30-0.39) and 3.25 (2.89-3.62) mmol/kg, respectively.
化学性质 白色针状结晶。熔点147-149℃,溶于醇、醚、丙酮,不溶于冷水。易溶于热水。无臭,味微苦。用途 该品具有镇静和轻度催眠作用,服后5分钟即显效,作用时间持续4h,无习惯性,可制成粉、片剂及注射剂。生产方法 以异戊醇为原料,先氧化生成异戊酸,再溴化生成α-溴代异戊酰溴,最后与尿素缩合生成溴异戊脲。类别 有毒物质毒性分级 中毒急性毒性 口服-大鼠 LD50: 1000 毫克/公斤; 口服-小鼠 LD50: 2000 毫克/公斤可燃性危险特性 热分解排出有毒溴化物和氮氧化物烟雾储运特性 库房通风低温干燥灭火剂 水,干粉、干砂、二氧化碳、泡沫、1211灭火剂
相关产品
|
||||||||||||||||||||||||||||||||||||
|
本网站展示的所有产品仅用于工业制造、技术研发、科学研究,所有产品非药品不可食用,依据国家相关法规及平台管理要求,购买相关危险物品应取得有效的资质、资格条件。 参考《应急管理部等多部门关于加强互联网销售危险化学品安全管理的通知 (应急〔2022〕119号)》 和《互联网危险物品信息发布管理规定》 Copyright © 2021-2025 chemhome123版权所有 |冀ICP备2024096099号- 1|冀公安备13042302000143号|互联网增值电信业务经营许可证:冀B2-20250121 |药品、 医疗器械互联网信息服务备案凭证备案号:(冀)-非经营性-2025-0099 |


 CAS号:26464-05-1
CAS号:26464-05-1 CAS号:57-13-6
CAS号:57-13-6 CAS号:503-74-2
CAS号:503-74-2 CAS号:123-51-3
CAS号:123-51-3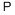 CAS号:7723-14-0
CAS号:7723-14-0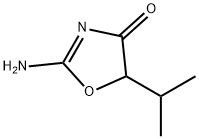 CAS号:15900-26-2
CAS号:15900-26-2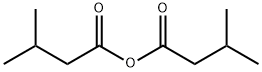 CAS号:1468-39-9
CAS号:1468-39-9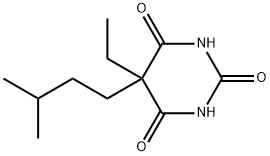 CAS号:57-43-2
CAS号:57-43-2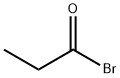 CAS号:598-22-1
CAS号:598-22-1 CAS号:110-46-3
CAS号:110-46-3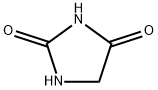 CAS号:461-72-3
CAS号:461-72-3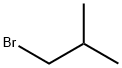 CAS号:78-77-3
CAS号:78-77-3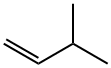 CAS号:563-45-1
CAS号:563-45-1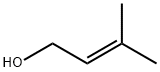 CAS号:556-82-1
CAS号:556-82-1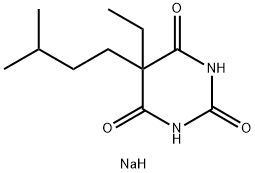 CAS号:64-43-7
CAS号:64-43-7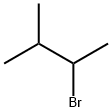 CAS号:18295-25-5
CAS号:18295-25-5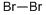 CAS号:7726-95-6
CAS号:7726-95-6