|
| ||||||||||||||||||||||||
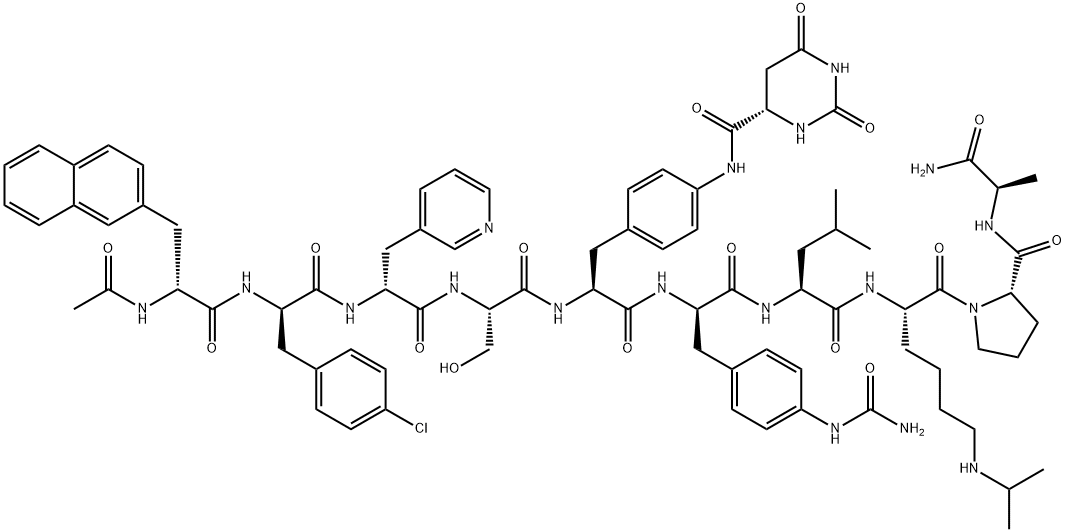
CAS号:214766-78-6
|
英文名称:Degarelix
分子式
C82H103ClN18O16
分子量
1632
EINECS号
807-277-4
MDL
MFCD05860888
Smiles
InChIKey
乙二醇化学百科
基本信息
物化性质
安全信息
生产及用途
醋酸地加瑞克是一种合成的促性腺激素释放激素(GnRH)受体拮抗剂,通过与脑下垂体的GnRH受体可逆的结合,减少促性腺激素及睾酮的释放,从而发挥抗前列腺癌作用。 地加瑞克(商品名:费蒙格®)是最新一代的GnRH拮抗剂,在其分子的5,6位并入了P-尿基苯丙氨酸,化学结构的改变使得药物作用时间延长的同时避免了因组胺释放引起的超敏反应。 地加瑞克是丹麦辉凌制药有限公司研发的促性腺激素释放激素(GnRH)受体抑制剂类药物,可逆性抑制垂体GnRH受体来减少促性腺激素释放继而抑制睾酮的释放。地加瑞克通过抑制对前列腺癌持续生长至关重要的睾酮来延缓前列腺癌的生长和恶化。以激素治疗前列腺癌来降低睾酮浓度的初期却造成睾酮浓度激增,此初始刺激该激素受体可暂时性促进肿瘤生长而不是抑制它,而地加瑞克则不会。 醋酸地加瑞克是一种促性腺激素释放激素受体阻滞剂,该药已分别在美国和欧美被批准用于晚期(激素依赖的)前列腺癌治疗,在日本被批准用于前列腺癌治疗。 Degarelix是一种竞争性,可逆的的促性腺激素释放激素受体 (GnRHR) 拮抗剂。GnRHR Degarelix acts directly on the pituitary receptors for luteinizing hormone-releasing hormone (LHRH), blocking the action of endogenous LHRH. The use of degarelix eliminates the initial undesirable surge in gonadotropin and testosterone levels, which is produced by agonists of LHRH. Degarelix treatment reduces cell viability in all prostate cell lines (WPE1-NA22, WPMY-1, BPH-1 cells, VCaP cells), with the exception of the PC-3 cells. The GnRH antagonist degarelix exerts a direct effect on prostate cell growth through apoptosis.
At single subcutaneous injections of 0.3 to 10 μg/kg in rats, degarelix produces a dose-dependent suppression of the pituitary-gonadal axis as revealed by the decrease in plasma luteinizing hormone (LH) and testosterone levels. Duration of LH suppression increases with the dose: in the rat, significant suppression of LH lasted 1, 2, and 7 days after a single subcutaneous injection of degarelix at 12.5, 50, or 200 μg/kg, respectively. Degarelix is stable when incubated in microsomes and cryopreserved hepatocytes from animal liver tissue. In rat and dog, most of the degarelix dose is eliminated within 48 h via urine and feces in equal amounts (40–50% in each matrix), whereas in monkey the major route of excretion is fecal (50%) and renal (22%).
参考质量标准 外观?白色粉末纯度(HPLC) ≥98.0%单杂≤1.0% 醋酸根含量5.0%~12.0% 水分含量≤10.0% 肽含量≥80.0%
上下游产品
上游产品共计:0个
下游产品共计:0个
相关产品
产品供应商
|
||||||||||||||||||||||||
|
本网站展示的所有产品仅用于工业制造、技术研发、科学研究,所有产品非药品不可食用,依据国家相关法规及平台管理要求,购买相关危险物品应取得有效的资质、资格条件。 参考《应急管理部等多部门关于加强互联网销售危险化学品安全管理的通知 (应急〔2022〕119号)》 和《互联网危险物品信息发布管理规定》 Copyright © 2021-2025 chemhome123版权所有 |冀ICP备2024096099号- 1|冀公安备13042302000143号|互联网增值电信业务经营许可证:冀B2-20250121 |药品、 医疗器械互联网信息服务备案凭证备案号:(冀)-非经营性-2025-0099 |


 CAS号:120287-85-6
CAS号:120287-85-6 CAS号:47931-85-1
CAS号:47931-85-1 CAS号:2434-49-3
CAS号:2434-49-3 CAS号:204656-20-2
CAS号:204656-20-2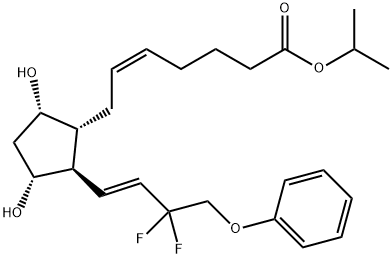 CAS号:209860-87-7
CAS号:209860-87-7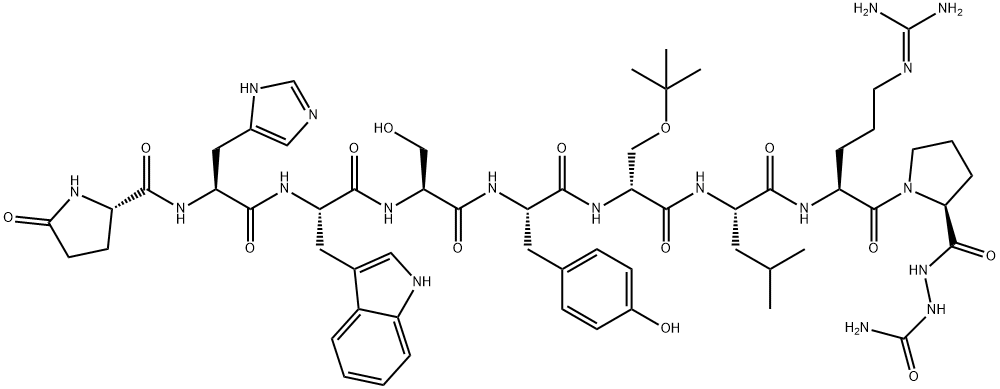 CAS号:65807-02-5
CAS号:65807-02-5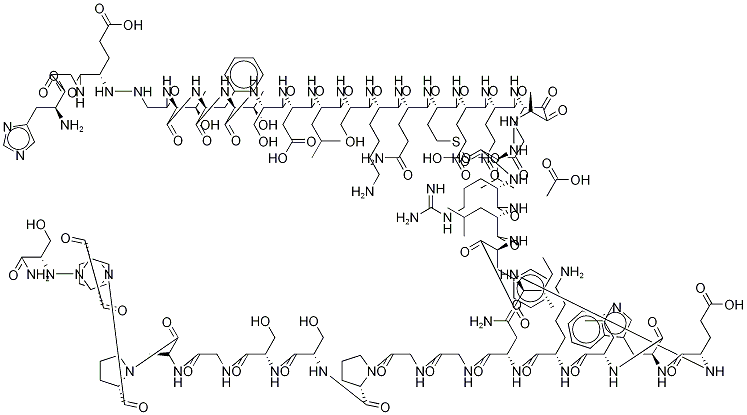 CAS号:141732-76-5
CAS号:141732-76-5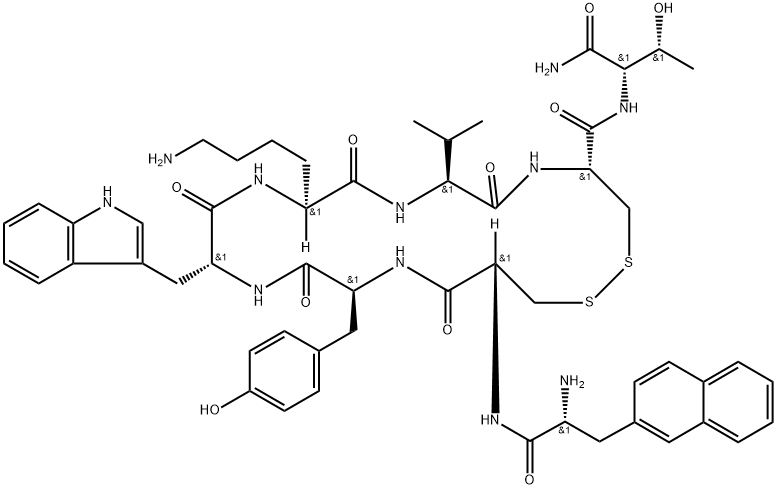 CAS号:108736-35-2
CAS号:108736-35-2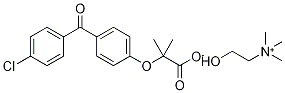 CAS号:856676-23-8
CAS号:856676-23-8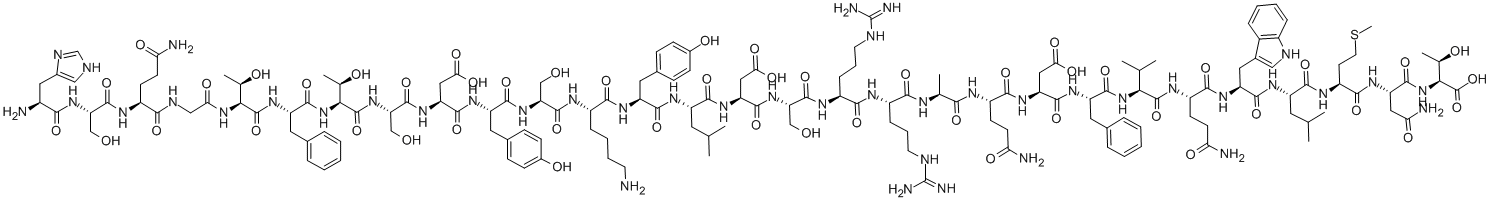 CAS号:16941-32-5
CAS号:16941-32-5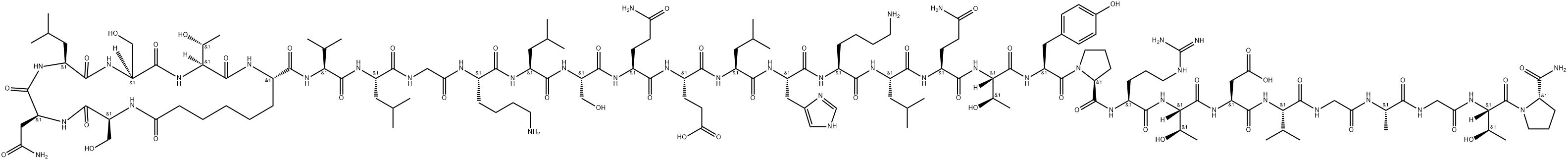 CAS号:60731-46-6
CAS号:60731-46-6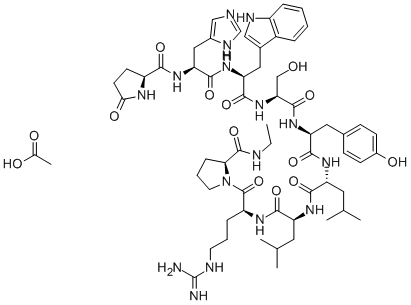 CAS号:53714-56-0
CAS号:53714-56-0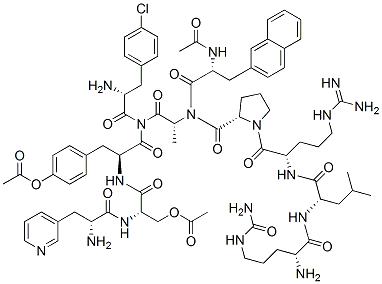 CAS号:130143-01-0
CAS号:130143-01-0