|
| ||||||||||||||||||||||||||||||||||||||||||||||||
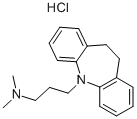
CAS号:113-52-0
|
英文名称:Imipramine hydrochloride
分子式
C19H25ClN2
分子量
317
EINECS号
204-030-7
MDL
MFCD00012669
Smiles
N1(CCCN(C)C)C2=CC=CC=C2CCC2C=CC=CC1=2.Cl
InChIKey
XZZXIYZZBJDEEP-UHFFFAOYSA-N
乙二醇化学百科
基本信息
物化性质
安全信息
生产及用途
Imipramine hydrochloride 抑制血清素转运蛋白(serotonin),IC50 值为 32 nM。Imipramine hydrochloride 可阻止胰酶的易位,抑制 MV 和外泌体的分泌。 IC50: 32 nM (serotonin) Depression-like behavior is often complicated by chronic pain. Antidepressants including imipramine are widely used to treat chronic pain, but the mechanisms are not fully understood. Imipramine (IC 50 =32 nM) and desipramine (IC 50 =160 nM) are found to be potent inhibitors of the human placental serotonin transporter.
Administration of imipramine reverses social avoidance behavior, significantly increasing the interaction time. 24 days of imipramine treatment in RSD mice significantly decreases stress-induced mRNA levels for IL-6 in brain microglia. Chronic mild stress induces a long-term altered gene expression profile in the prefrontal cortex that is partially reverted by imipramine treatment (10mg/kg, i.p.). Chronic imipramine administration alteres the amino acid dynamics in the brain. In the striatum, the concentrations of asparagine, glutamine and methionine are significantly increased by chronic imipramine administration. In the thalamus and hypothalamus, chronic imipramine administration significantly decreased the valine concentration. Imipramine reduces pain-related negative emotion without influencing pain and that this effect is diminished by denervation of 5-HT neurons and by anti-BDNF treatment. Imipramine also normalizes derangement of ERK/CREB coupling, which leads to induction of BDNF. This suggests a possible interaction between 5-HT and BDNF. Imipramine treatment counteracts the corticosterone administration-induced increase in the reactivity of rat CA3 hippocampal circuitry to the activation of the 5-HT receptor.
化学性质 白色结晶性粉末。熔点174-175℃。易溶于水、乙醇,微溶于丙酮,几乎不溶于乙醚。无臭,味苦。遇光渐变色。米帕明碱([50-49-7])沸点160℃(13.3Pa)。用途 镇静安眠性抗组织胺及抗抑郁药,治疗精神抑郁症及小儿遗尿症。用途 用于治疗各种类型抑郁症、小儿遗尿症等生产方法 10,11-二氢-5-二苯并[b,f]氮杂卓(见14230)与甲苯、钠氨一起加热回流1h,冷至40-50℃,滴加1-氯-3-二甲氨基丙烷,再回流16h。冷却,过滤,滤液水洗层,取甲苯层减压蒸馏,回收甲苯后,收集210-230℃(0.67kPa)馏分,得米帕明碱,最后经成盐而得成品。
相关产品
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
本网站展示的所有产品仅用于工业制造、技术研发、科学研究,所有产品非药品不可食用,依据国家相关法规及平台管理要求,购买相关危险物品应取得有效的资质、资格条件。 参考《应急管理部等多部门关于加强互联网销售危险化学品安全管理的通知 (应急〔2022〕119号)》 和《互联网危险物品信息发布管理规定》 Copyright © 2021-2025 chemhome123版权所有 |冀ICP备2024096099号- 1|冀公安备13042302000143号|互联网增值电信业务经营许可证:冀B2-20250121 |药品、 医疗器械互联网信息服务备案凭证备案号:(冀)-非经营性-2025-0099 |


 CAS号:50-49-7
CAS号:50-49-7 CAS号:111-49-9
CAS号:111-49-9 CAS号:494-19-9
CAS号:494-19-9 CAS号:7647-01-0
CAS号:7647-01-0 CAS号:7782-92-5
CAS号:7782-92-5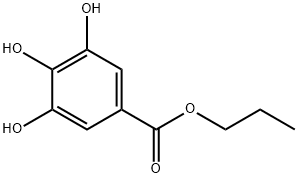 CAS号:121-79-9
CAS号:121-79-9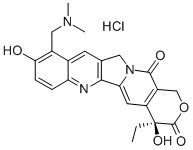 CAS号:119413-54-6
CAS号:119413-54-6 CAS号:7681-67-6
CAS号:7681-67-6 CAS号:84485-00-7
CAS号:84485-00-7 CAS号:33817-09-3
CAS号:33817-09-3 CAS号:739-71-9
CAS号:739-71-9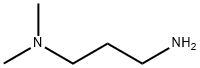 CAS号:109-55-7
CAS号:109-55-7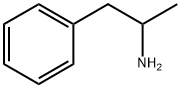 CAS号:300-62-9
CAS号:300-62-9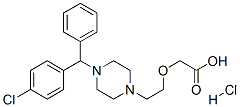 CAS号:83881-52-1
CAS号:83881-52-1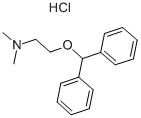 CAS号:147-24-0
CAS号:147-24-0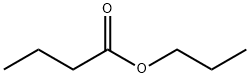 CAS号:105-66-8
CAS号:105-66-8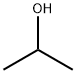 CAS号:67-63-0
CAS号:67-63-0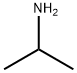 CAS号:75-31-0
CAS号:75-31-0